 心输出量和脑血流
心输出量和脑血流
# 心输出量和脑血流:成人脑灌注的综合调节
Cardiac Output and Cerebral Blood Flow: The Integrated Regulation of Brain Perfusion in Adult Humans
Lingzhong Meng, Wugang Hou, Jason Chui, Ruquan Han, Adrian W. Gelb; Cardiac Output and Cerebral Blood Flow: The Integrated Regulation of Brain Perfusion in Adult Humans. Anesthesiology 2015; 123:1198–1208 doi: https://doi.org/10.1097/ALN.0000000000000872
DeepL 翻译 + 人工校对# 摘要
Abstract
Cerebral blood flow (CBF) is rigorously regulated by various powerful mechanisms to safeguard the match between cerebral metabolic demand and supply. The question of how a change in cardiac output (CO) affects CBF is fundamental, because CBF is dependent on constantly receiving a significant proportion of CO. The authors reviewed the studies that investigated the association between CO and CBF in healthy volunteers and patients with chronic heart failure. The overall evidence shows that an alteration in CO, either acutely or chronically, leads to a change in CBF that is independent of other CBF-regulating parameters including blood pressure and carbon dioxide. However, studies on the association between CO and CBF in patients with varying neurologic, medical, and surgical conditions were confounded by methodologic limitations. Given that CBF regulation is multifactorial but the various processes must exert their effects on the cerebral circulation simultaneously, the authors propose a conceptual framework that integrates the various CBF-regulating processes at the level of cerebral arteries/arterioles while still maintaining autoregulation. The clinical implications pertinent to the effect of CO on CBF are discussed. Outcome research relating to the management of CO and CBF in high-risk patients or during high-risk surgeries is needed.
脑血流(CBF)受到各种强大机制的严格调节,以保障脑代谢供需匹配。心输出量(CO)的变化如何影响 CBF 的问题是很重要的,因为 CBF 依赖于持续不断的接受到相当大比例的 CO。作者回顾了调查健康志愿者和慢性心力衰竭患者的 CO 和 CBF 之间关系的研究。总体证据显示,急性或慢性的 CO 改变会导致 CBF 的改变,这种改变与其他 CBF 调节参数(包括血压和二氧化碳)无关。然而,对不同神经系统、内科和外科疾病患者的 CO 和 CBF 之间的关系的研究被方法学的局限性所混淆。鉴于 CBF 调节是多因素的,但各种过程必须同时对脑循环产生影响,作者提出了一个概念框架,在脑动脉 / 小动脉层面整合各种 CBF 调节过程,同时仍保持自动调节。讨论了与 CO 对 CBF 的影响有关的临床意义。需要对高危患者或高危手术期间的 CO 和 CBF 的管理相关预后进行研究。
Cardiac output causally affects cerebral blood flow. A conceptualization is proposed for the purpose of integrating at the level of cerebral resistance vessel various mechanisms that regulate the cerebral circulation and jointly determine the brain perfusion.
心输出量对脑血流的影响是因果关系。提出了一个概念,目的是在脑阻力血管的水平上整合调节脑循环的各种机制,共同决定脑灌注。
# 序言
THE brain, as a vital organ, disproportionately receives about 12% of cardiac output (CO) even though it weighs only 2% of the body weight.[1] Cerebral blood flow (CBF) is regulated by a set of powerful mechanisms that include cerebral autoregulation,[2] neurovascular coupling,[3] and cerebrovascular carbon dioxide and oxygen reactivity.[4] It is common to presume that a stable blood pressure or a fluctuating blood pressure as long as it is within the autoregulatory range will not lead to a noticeable change in CBF according to cerebral autoregulation. However, evidence shows that, even though the blood pressure remains stable or within the autoregulatory range, an alteration in CO, either acutely[5–9] or chronically,[10–19] leads to a change in CBF. Thus, it is pertinent to understand the effect of CO on CBF within the framework of cerebral autoregulation, a mechanism describing the effect of cerebral perfusion pressure on CBF.
大脑作为一个重要的器官,尽管它只占体重的 2%,却不成比例地接受了约 12% 的心输出量(CO)。[1] 脑血流(CBF)由一系列强大的机制调节,包括脑自动调节,[2] 神经血管耦合,[3] 和脑血管的二氧化碳和氧的反应性。 [4] 人们普遍认为,只要血压在自动调节范围内,稳定的血压或波动的血压只要在大脑自动调节范围内就不会导致 CBF 的明显变化。然而,有证据表明,即使血压保持稳定或在自动调节范围内,CO 的改变,无论是急性的 [5-9] 还是慢性的,[10-19] 都可导致 CBF 的变化。因此,在大脑自动调节的框架内理解 CO 对 CBF 的影响是关键的,这是一种描述脑灌注压对 CBF 影响的机制。
Optimal organ perfusion is fundamental to avoiding tissue ischemia and overperfusion. There are two common theories to explain the relationship between organ perfusion and systemic hemodynamics. The first is based on an analogy to Ohm’s law: organ perfusion depends on arterial blood pressure and vascular resistance of the organ. The other is based on the distribution of CO: the blood flow of each organ is a portion of CO that is determined by the value of CO and the percentage of share based on the organ’s metabolic need.[1]
最佳的器官灌注是避免组织缺血和过度灌注的基础。有两种常见的理论来解释器官灌注和系统血流动力学之间的关系。第一种是基于对欧姆定律的类比:器官灌注取决于动脉血压和器官的血管阻力。另一个是基于 CO 的分布:每个器官的血流是 CO 的一部分,由 CO 的值和基于器官代谢需要的份额比例决定。[1]
The effect of CO on CBF is a topic that has not been reviewed specifically. However, it is a clinically relevant issue because both acute and chronic changes in CO are frequently encountered in clinical care. In addition, it seems that a revision of the traditional framework of cerebral autoregulation is needed to integrate the effects of cerebral perfusion pressure and CO on brain perfusion in one concordant context. This is an important consideration because blood pressure and CO are related but different systemic hemodynamic parameters, and they usually change simultaneously and may exert distinctive effects on brain perfusion.[20]
CO 对 CBF 的影响是一个还没有专门综述的话题。然而,这是一个与临床相关的问题,因为 CO 的急性和慢性变化在临床护理中都经常遇到。此外,似乎需要对传统的脑自动调节框架进行修订,以便将脑灌注压和 CO 对脑灌注的影响整合在一个统一的的背景下。这是一个重要的考虑因素,因为血压和 CO 是相关但又是不同的全身血液动力学参数,它们通常同时变化,并可能对脑灌注产生不同的影响。[20]
The aims of this review are (1) to examine the evidence of the association between CO and CBF under varying conditions in adult humans, (2) to present a revised conceptual framework that integrates different regulatory mechanisms of brain perfusion, and (3) to discuss the relevant clinical implications.
本综述的目的是:(1)研究成人不同条件下 CO 和 CBF 之间关联的证据,(2)提出一个修订的概念框架,整合脑灌注的不同调节机制,以及(3)讨论相关的临床意义。
# CO 的急性变化对 CBF 的影响
Effect of Acute Change in CO on CBF
# 证据
Evidence
A distinct association between CO and CBF was demonstrated in young healthy volunteers whose central blood volume was decreased via lower body negative pressure[5],[7–9] or standing up[6] and increased via leg tensing,[6] albumin infusion,[8] or normal saline infusion[9] ([table 1]). Each percentage change in CO corresponded to a 0.35% change in CBF, that is, there is about a 10% CBF decrease for a 30% CO reduction based on eight data pairs from five previous studies (R2 = 0.9, [fig. 1]). This association was unlikely to have been confounded by a change in either blood pressure or carbon dioxide because both parameters remained relatively stable except two studies in which carbon dioxide had a clinically significant drop after standing up[6] and lower body negative pressure,[7] respectively. It was also unlikely to be ascribed to a change in cerebral metabolic activity, because these studies were done in resting and unanesthetized subjects. Therefore, the association between CO and CBF is a causal relationship. The finding that β1-adrenergic blockade concurrently attenuated the increase in both CO and CBF induced by cycling corroborates this proposition.[21]
在年轻的健康志愿者中,通过下体负压[1] [5]、[7-9] 或站立 [6] 减少中心血容量,通过腿部加压、[6] 白蛋白输注、[8] 或正常盐水输注等方式,证明 CO 增加与 CBF 之间存在明显关联(【表 1】。CO 每变化 1% 对应于 CBF 变化 0.35%,也就是说,根据以往 5 项研究的 8 个数据对,CO 减少 30%,CBF 大约减少 10%(R2=0.9,[图 1])。这种关联不太可能被血压或二氧化碳的变化所混淆,因为除了两项研究中二氧化碳在站立后 [6] 和下体负压后分别有临床意义上的下降外,这两项参数都保持相对稳定。这也不太可能归因于脑代谢活动的变化,因为这些研究是在静息的和未麻醉的受试者中进行的。因此,CO 和 CBF 之间的关联是一种因果关系。β1 - 肾上腺素能阻断可同时减弱了骑车引起的 CO 和 CBF 的增加的结果,证实了这一主张。[21]
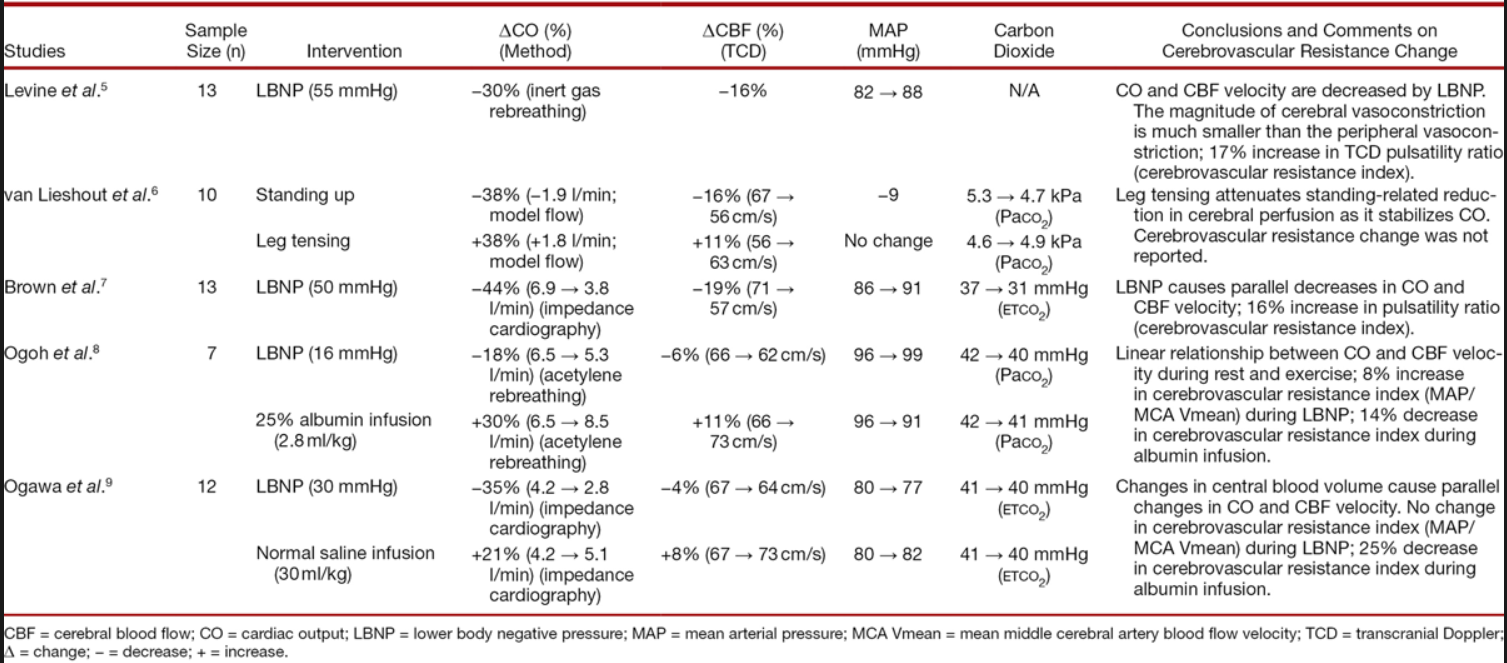
Table 1. Studies Investigating Simultaneous Changes in Cardiac Output and Cerebral Blood Flow via Central Blood Volume Alteration in Unanesthetized Healthy Volunteers
表 1. 调查未麻醉的健康志愿者通过中心血容量改变同时改变心输出量和脑血流量的研究
Fig. 1. 图 1.
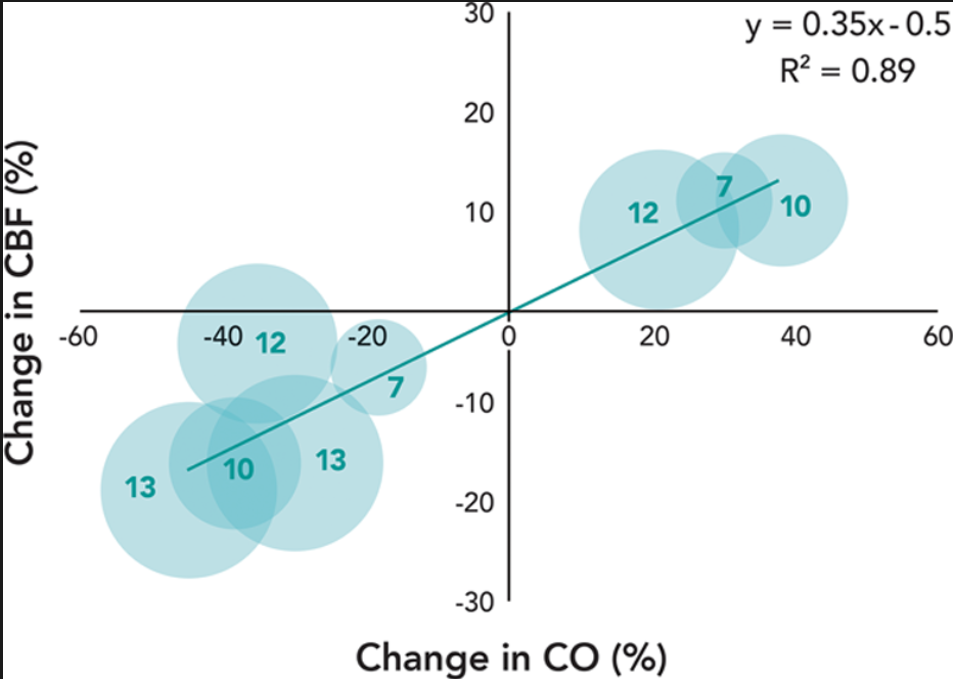
Correlation between changes in cardiac output (CO) and cerebral blood flow (CBF) in unanesthetized healthy volunteers during central blood volume alterations.[5–9] All data are reported in [table 1]. The diameter of the dot represents the sample size that is also indicated by the number inside of each dot.
未麻醉的健康志愿者在中心血容量改变过程中心输出量(CO)和脑血流量(CBF)变化的相关性。[5-9] 所有数据都在 [表 1] 中报告。点的直径代表样本量,也由每个点 * 内的数字表示。
However, differences among the methodologies used to alter CO and measure CO and CBF should be considered during data interpretation. In these studies, the CO was altered via an acute change in central blood volume using different maneuvers, and the CO was measured using different methods even though the CBF was always assessed using transcranial Doppler (TCD; [table 1]). There is a chance that methodologic heterogeneity could cause inconsistent results. In addition, the practice of using TCD-measured middle cerebral artery blood flow velocity as a CBF surrogate has been cautioned against, especially in patients with cerebrovascular diseases.[22],[23]
然而,在数据解释过程中应考虑用于改变 CO 和测量 CO 和 CBF 的方法之间的差异。在这些研究中,通过使用不同的手法导致中心血容量的急性变化来改变 CO,并且使用不同的方法测量 CO,即使 CBF 总是使用经颅多普勒(TCD; [表 1])来评估。方法上的异质性有可能导致结果不一致。此外,使用 TCD 测量的大脑中动脉血流速度作为 CBF 替代物的做法已被提醒要小心,特别是在脑血管疾病患者中。[22],[23]
In contrast, a recent study failed to define an association between cardiac index and CBF with both parameters measured using magnetic resonance imaging techniques in 31 healthy subjects of 50 to 75 yr.[24] There are a multitude of differences between this study and the previous studies summarized in [table 1]. The most prominent is that the CO (and CBF as a consequence) was not acutely altered compared with that of the previous studies. It is worth noting that the fractional CBF, defined as the ratio of CBF to CO, was inversely correlated with cardiac index (R2 = 0.22, P = 0.008), implying that when the CO is decreased, the brain shares an increasing percentage of CO.[24]
相反,最近的一项研究未能确定心脏指数和 CBF 之间的关联,这两个参数是在 31 名 50 至 75 岁的健康受试者中用磁共振成像技术测量的。[24] 这项研究与 [表 1] 中总结的以往研究有许多不同之处。最突出的是,与以前的研究相比,CO(和 CBF 的结果)没有急性改变。值得注意的是,部分 CBF(定义为 CBF 与 CO 的比率)与心脏指数成反比(R2=0.22,P=0.008),这意味着当 CO 减少时,大脑分享的 CO 的比例越来越高。
# 机制
Mechanism
When the CBF was changed during acute central blood volume alteration, there must be a change in cerebrovascular resistance to account for the flow change because the blood pressure remained relatively stable. Indeed, three of four studies showed an increase in cerebrovascular resistance assessed using TCD pulsatility ratio during lower body negative pressure,[5],[7],[8] and two studies showed a decrease during albumin or normal saline infusion.[8],[9] The common causes of a change in cerebrovascular resistance are (1) a change in cerebral perfusion pressure via autoregulation,[2] (2) a change in cerebral metabolic activity via neurovascular coupling,[3] (3) a change in arterial blood carbon dioxide partial pressure via ventilation change,[25] and (4) a change in sympathetic nervous activity via the sympathetic innervation of the cerebral resistance vessels.[26] The first three options are essentially excluded based on the study conditions.[5],[7–9] Therefore, by exclusion, this attributes the increase in cerebrovascular resistance to the sympathoexcitation incurred by central blood volume alteration.[8]
当 CBF 在急性中心血容量改变时,由于血压保持相对稳定,必须有脑血管阻力的变化来说明血流变化。事实上,四项研究中的三项显示,在下半身负压时,用 TCD 脉动率评估的脑血管阻力增加,[5], [7], [8],两项研究显示在输注白蛋白或正常盐水时,脑血管阻力下降。 [8],[9] 导致脑血管阻力变化的常见原因有:(1)脑灌注压力的变化是通过自动调节 (2)脑代谢活动的变化是通过神经血管耦合 [3],(3) 动脉血二氧化碳分压的变化是通过通气变化 [25] ,(4) 交感神经活动的变化是通过脑阻力血管的交感神经支配。 [26] 基于研究条件,前三个选项基本被排除。[5], [7-9] 因此,通过排除法,这将脑血管阻力的增加归因于中心血容量改变所引起的交感神经兴奋。
During acute central blood volume alteration, the extent of the CBF change is much smaller (about one third) than the change in peripheral regional blood flow.[5],[8] This may be because of either the relatively minor role the sympathetic nervous system plays in the brain perfusion compared with the periphery[26–29] or the countering effects by other robust CBF-regulating mechanisms that the periphery lacks. Physiologically, the differential extent of vasoconstriction in different vascular beds shunts the flow from the periphery to the brain because brain perfusion is a priority during acute CO reduction.
在急性中枢血容量改变期间,CBF 的变化程度比外周区域血流的变化要小得多(大约三分之一)。[5], [8] 这可能是因为交感神经系统在脑灌注中发挥的作用与外周相比相对较小 [26-29] 或外周缺乏其他强大的 CBF 调节机制的反作用。在生理上,不同血管床的血管收缩程度不同,将流量从外围分流到大脑,因为在急性 CO 减少期间,大脑灌注是优先考虑的。
However, direct evidence of how the simultaneous acute changes in CO and CBF are mediated by the sympathetic nervous system is lacking, and therefore, the mechanism(s) responsible for the acute change in CBF because of an acute change in CO remains largely speculative.
然而,缺乏直接证据表明 CO 和 CBF 的同时急性变化是如何由交感神经系统介导的,因此,由于 CO 的急性变化而导致 CBF 急性变化的机制在很大程度上仍然是推测的。
# CO 慢性下降对 CBF 的影响
Effect of Chronically Reduced CO on CBF
# 证据
Evidence
Extensive evidence shows that CBF is reduced in patients diagnosed with chronic heart failure compared with that of control who do not have cardiac insufficiency ([table 2]).[10–19] The extent of the CBF reduction correlates with the severity of the chronic heart failure assessed using New York Heart Association functional classification[14] and left ventricular ejection fraction.[18] The CBF reduction is reversed by interventions including cardiac transplantation,[13–15] cardiac resynchronization therapy,[17],[19] cardioversion,[30] and captopril treatment[10–12] ([fig. 2]). Overall, a causal relationship between CO and CBF in patients with chronic heart failure is implied. This proposition is corroborated by a recent study that showed an exaggerated cerebral hypoperfusion in the upright posture in patients with heart failure compared with age- and sex-matched healthy controls.[31]
大量证据表明,与没有心功能不全的对照组相比,被诊断为慢性心力衰竭的患者的 CBF 减少([表 2])。[10-19] CBF 减少的程度与使用纽约心脏协会功能分类 [14] 和左心室射血分数评估的慢性心衰的严重程度相关。 [18] CBF 的减少被干预措施逆转,包括心脏移植、[13-15] 心脏再同步化治疗、[17]、[19] 心脏复律、[30] 和卡托普利治疗 [10-12] ([fig. 2](javascript: 😉)。总的来说,慢性心力衰竭患者的 CO 和 CBF 之间的因果关系是暗示的。最近的一项研究证实了这一观点,该研究显示,与年龄和性别匹配的健康对照组相比,心力衰竭患者在直立姿势下的脑灌注不足是加重的。
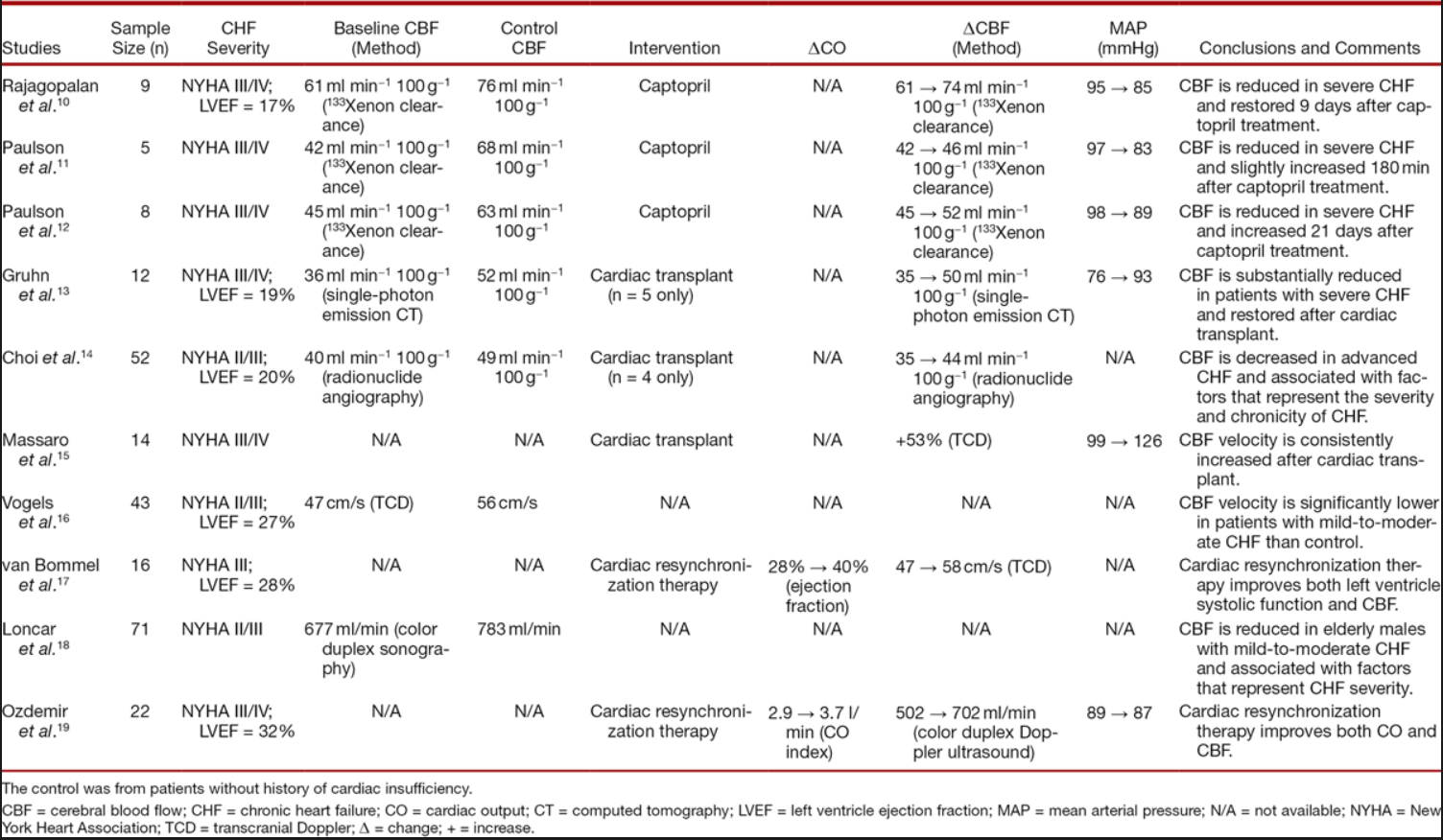
Table 2. Cerebral Blood Flow at Baseline and/or after Various Interventions in Patients with Chronic Heart Failure
表 2. 慢性心力衰竭患者基线和 / 或各种干预措施后的脑血流情况
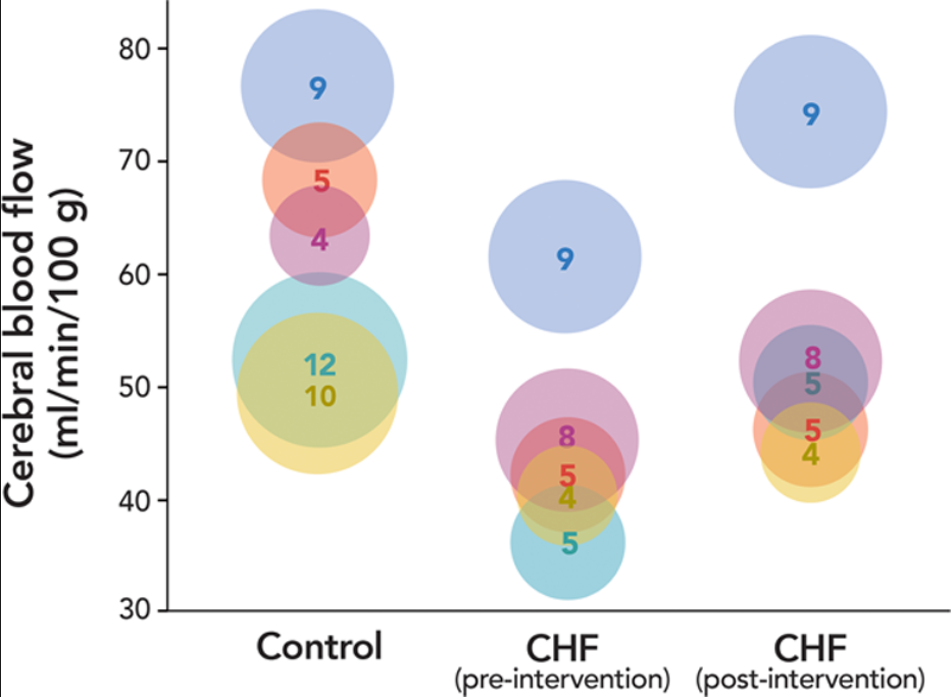
Fig. 2. Cerebral blood flow in patients diagnosed with chronic heart failure (CHF) before (preintervention) and after (postintervention) various interventions.[10–14] The control was from patients without cardiac insufficiency. All data are reported in [table 2]. Only the studies that reported the control, preintervention, and postintervention values of cerebral blood flow in the units of ml min-1 100 g-1 were included. The dots with the same color are from the same study. The diameter of the dot represents the sample size that is also indicated by the number inside of each dot.
图 2. 被诊断为慢性心力衰竭(CHF)的患者在各种干预措施之前(干预前)和之后(干预后)的脑血流量。[10-14] 对照组来自没有心功能不全的患者。所有数据都在 [表 2] 中报告。只包括报告了对照组、干预前和干预后的脑血流值的研究,单位为 ml min-1 100 g-1。颜色相同的点是来自同一研究。点的直径代表样本量,也由每个点内的数字表示。
However, the methodologic heterogeneity and limitations of these studies should be recognized. The sample size in the intervention studies, especially those with cardiac transplantation, was small.[13–15] The increased blood pressure after cardiac transplantation can confound the interpretation of the effect of improved CO on CBF.[13],[15] In rodents, captopril treatment can further reduce the lower limit of cerebral autoregulation after nephrectomy,[32] decrease infarction size via CBF improvement after ischemic stroke,[33] and restore cerebral autoregulation after hemorrhagic stroke.[34] Therefore, studies with captopril treatment can be confounded by the direct effect of captopril on CBF regulation.[10–12]
然而,应该认识到这些研究的方法学异质性和局限性。干预研究的样本量很小,尤其是那些有心脏移植的研究。[13-15] 心脏移植后血压升高会混淆改善 CO 对 CBF 影响的解释。 [13],[15] 在啮齿动物中,卡托普利治疗可以进一步降低肾切除术后脑自动调节的下限,[32] 通过改善缺血性卒中后的 CBF,减少梗死面积,[33] 并恢复出血性卒中后脑自动调节。 [34] 因此,用卡托普利治疗的研究可能会被卡托普利对 CBF 调节的直接作用所混淆。
# 机制
Mechanism
The mechanism underlying the CBF reduction in patients with chronic heart failure is unclear but likely related to the neurohormonal activation incurred by a failing heart. The hyperactivity of both the sympathetic nervous system and the renin–angiotensin–aldosterone axis provokes vasoconstriction of not only the peripheral vascular beds but also the cerebral vascular bed.[35–38] The circulating and locally formed angiotensin II may contribute to the decrease of CBF via the AT1 receptors expressed in cerebrovascular endothelial cells and in the brain regions controlling cerebral circulation.[39] Similar to the effect of acute CO reduction on CBF, the differential extent of vasoconstriction of different vascular beds shunts the flow from the periphery to the brain in patients with chronic heart failure, resulting in a lesser extent of CBF reduction than both the CO and the peripheral blood flow.[35]
慢性心力衰竭患者 CBF 减少的机制尚不清楚,但可能与心脏衰竭引起的神经荷尔蒙激活有关。交感神经系统和肾素 - 血管紧张素 - 醛固酮轴的过度活跃不仅引起外周血管床的血管收缩,也引起脑血管床的血管收缩。[35-38] 循环和局部形成的血管紧张素 II 可能通过脑血管内皮细胞和控制脑循环的脑区表达的 AT1 受体(血管紧张素 1 型受体)促进 CBF 的减少。 [39] 与急性 CO 减少对 CBF 的影响类似,不同血管床的血管收缩程度不同,在慢性心力衰竭患者中,从外周分流到大脑,导致 CBF 减少的程度比 CO 和外周血流都小。
# 神经认知功能损害
Neurocognitive Impairment
A relevant question that deserves discussion is what the consequences of the reduced cerebral perfusion are in patients with chronic heart failure. It is counterintuitive to assume that long-term suboptimal brain perfusion is inconsequential. Indeed, abundant evidence shows that the prevalence of cognitive dysfunction is inappropriately high in patients diagnosed with chronic heart failure.[40–45] The odds ratio for cognitive impairment in patients with chronic heart failure is 1.62 with the 95% CI of 1.48 to 1.79 (P <0.0001) based on a systematic review.[43] The extent of cognitive impairment parallels the severity of chronic heart failure.[42],[44],[46] Both cardiac resynchronization therapy[47],[48] and transplantation[49],[50] improved the impaired cognition.
一个值得讨论的相关问题是慢性心力衰竭患者脑灌注减少的后果是什么。长期的脑灌注不足是无足轻重的假设是反直觉的。事实上,大量证据显示,在被诊断为慢性心力衰竭的患者中,认知功能障碍的发生率异常高。[40-45] 根据一项系统回顾,慢性心力衰竭患者认知功能障碍的几率为 1.62,95% CI 为 1.48 至 1.79(P<0.0001)。 [43] 认知障碍的程度与慢性心力衰竭的严重程度相似。[42], [44], [46] 心脏再同步化治疗 [47], [48] 和移植手术 [49], [50](javascript: ;) 都能改善受损的认知能力。
Chronic heart failure is also linked to abnormal brain aging and Alzheimer disease.[51–54] The relentless cerebral hypoperfusion and neurohormonal hyperactivity likely contribute to the dysfunction of the neurovascular unit.[53],[54] The neuronal energy crisis facilitates protein synthesis abnormalities that include impaired clearance of amyloid β and hyperphosphorylation of τ protein, ending up with the formation of amyloid-β plaques and neurofibrillary tangles.[54],[55]
慢性心力衰竭也与大脑异常老化和阿尔茨海默病有关。[51-54] 持续的大脑低灌注和神经激素过度活跃很可能导致神经血管单元的功能紊乱。 [53],[54] 神经元的能量危机促进了蛋白质合成的异常,包括淀粉样 β 的清除障碍和 τ 蛋白的过度磷酸化,最终形成淀粉样 β 斑块和神经纤维缠结。
Despite the plausible notion that there is a link among chronic heart failure, cerebral hypoperfusion, and neurocognitive dysfunction, caution is needed before claiming a causal relationship because these chronic conditions share risk factors. In addition, not every patient with neurocognitive impairment has chronic heart failure and vice versa.
尽管慢性心力衰竭、脑灌注不足和神经认知功能障碍之间存在着合理的概念,但在声称有因果关系之前需要谨慎,因为这些慢性疾病有共同的风险因素。此外,并非每个神经认知障碍的患者都有慢性心力衰竭,反之亦然。
# 显示 CO-CBF 有关联的疾病状态
Disease States Demonstrating CO–CBF Association
# 血管痉挛
Vasospasm
The goal in treating vasospasm induced by subarachnoid hemorrhage is to restore the reduced CBF. One of the strategies is to augment the CO with the hope of improving the cerebral perfusion. A clinical study found that a 46% increase in CO via dobutamine infusion led to a significant increase in CBF (from 25 to 35 ml min-1 100 g-1) in the brain regions perfused by the vasospastic arteries.[56] The increase in cerebral perfusion took place despite a decrease in mean arterial pressure from 113 to 108 mmHg. The result of this study was corroborated in a separate study that showed the clinical reversal of the ischemic symptoms by dobutamine infusion combined with hypervolemic preloading in 78% of symptomatic patients.[57] Intraaortic balloon pump counterpulsation has also been tested in this patient population. In a report of 15 cases in which this treatment was used in patients who also had neurogenic stress cardiomyopathy, it was concluded that the use of intraaortic balloon pump counterpulsation was effective in preventing the delayed ischemic neurologic deficits.[58]
治疗蛛网膜下腔出血引起的血管痉挛的目标是恢复降低的 CBF。其中一个策略是增加 CO,希望能改善脑灌注。一项临床研究发现,通过多巴胺输注,CO 增加 46%,导致由血管痉挛动脉灌注的脑区的 CBF 显著增加(从 25 到 35 ml min-1 100 g-1)。[56] 尽管平均动脉压从 113 毫米汞柱下降到 108 毫米汞柱,脑灌注的增加还是发生了。这项研究的结果在另一项研究中得到了证实,该研究显示,78% 的有症状的病人通过多巴胺输注结合高血容量预负荷,使缺血症状得到了临床逆转。在一份对 15 个病例的报告中,对同时患有神经源性应激性心肌病的患者使用了这种治疗方法,结论是使用主动脉内球囊反搏能有效地防止延迟性缺血性神经功能障碍。
# 缺血性卒中
Ischemic Stroke
In patients with acute ischemic stroke in the middle cerebral artery territory, an association between CO and TCD-estimated CBF was demonstrated in the affected, but not the unaffected, brain region when using hypervolemic hemodilution combined with dopamine–dobutamine infusions.[59] Intraaortic balloon pump counterpulsation was also found to increase TCD-estimated CBF by 21 and 11% in patients with acute ischemic stroke whose left ventricular ejection fractions were 28 and 44%, respectively.[60] Intraaortic balloon pump counterpulsation normally decreases systolic blood pressure, increases diastolic blood pressure, and produces little or no change in mean blood pressure in normotensive patients.[61] Therefore, it is reasonable to attribute the improvement of the CBF to the augmentation of the CO during the application of intraaortic balloon pump counterpulsation.
在大脑中动脉区域的急性缺血性卒中患者中,使用高血容量血液稀释法结合多巴胺 - 多巴酚丁胺输注时,在受影响的脑区,而不是未受影响的脑区,证明了 CO 和 TCD 估计的 CBF 之间存在关联。 [59] 还发现在左心室射血分数为 28% 和 44% 的急性缺血性卒中患者中,主动脉内球囊反搏可使 TCD 估算的 CBF 分别增加 21% 和 11%。 [60] 主动脉内球囊反搏通常会降低收缩压,增加舒张压,在血压正常的病人中,平均血压几乎没有变化。[61] 因此,将 CBF 的改善归因于应用主动脉内球囊反搏时 CO 的增强是合理的。
# 脓毒症
Sepsis
In studies conducted in septic patients, dobutamine infusion increased both cardiac index (from 3.4 to 4.2 L min-1 m-2 and from 3.8 to 6.3 L min-1 m-2) and TCD-estimated CBF (from 52 to 62 cm/s and from 68 to 80 cm/s), whereas the increase in mean arterial pressure was from 85 to 91 mmHg and from 77 to 86 mmHg, respectively.[62],[63] Both studies showed a better correlation between CO and CBF than between blood pressure and CBF using both the relative changes of parameters[63] and the absolute values of measurements.[62]
在对脓毒症患者进行的研究中,多巴胺输注使心脏指数(从 3.4 到 4.2 L min-1 m-2 和从 3.8 到 6.3 L min-1 m-2)和 TCD 估计的 CBF(从 52 到 62 cm/s 和从 68 到 80 cm/s)都有所增加,而平均动脉压的增加分别从 85 到 91mmHg 和从 77 到 86mmHg。 [62], [63] 两项研究都显示,使用参数的相对变化 [63] 和测量的绝对值,CO 和 CBF 之间的相关性优于血压和 CBF 之间的相关性。
# 显示 CO-CBF 缺乏关联的疾病状态
Disease States Demonstrating a Lack of CO–CBF Association
# 脑外伤
Head Injury
An association between changes in CO and CBF (133Xe washout) was not found during treatment with phenylephrine, trimethaphan or mannitol in comatose and ventilated patients with severe head injury.[64] Phenylephrine, which is a peripheral vasoconstrictor used to increase blood pressure, actually causes a decrease in CO.[20] This may have confounded the study. An increase in perfusion pressure can lead to an increase in CBF in neurologically critically ill patients who have impaired autoregulation[65] ; as a result, phenylephrine treatment likely causes opposite changes in CBF (increase) and CO (decrease).
在用苯肾上腺素、曲美沙芬或甘露醇治疗昏迷和机械通气的严重颅脑损伤患者时,没有发现 CO 和 CBF(133Xe 冲洗)变化之间的关联。[64] 苯肾上腺素是一种用于增加血压的外周血管收缩剂,实际上会导致 CO 下降。这可能对研究产生混杂影响。灌注压的增加可导致自动调节功能受损的神经系统重症患者的 CBF 增加 [65] ;因此,苯肾上腺素治疗可能会导致 CBF(增加)和 CO(减少)的相反变化。
# 神经系统手术
Neurologic Surgery
CBF is normally increased after surgical resection of brain arteriovenous malformations. However, an association between changes in CO and CBF (133Xe washout) based on the preresection and postresection measurements was not found in this patient population.[66] Hemodynamic variables including CO, arterial blood pressure, central venous pressure, and pulmonary artery diastolic pressure remained stable in the face of the increase in CBF. Brain arteriovenous malformations have unique hemodynamic physiology including the relatively low transnidal pressure gradient that may shunt a portion of CBF through the lesion.[67] Thus, it is speculated that after surgical resection, the portion of CBF originally going through the arteriovenous malformation reroutes through the normal brain resulting in a regionally increased CBF in the face of unchanged systemic hemodynamics.
脑动静脉畸形的手术切除后,CBF 通常会增加。然而,根据切除前和切除后的测量结果,在这个病人群体中没有发现 CO 和 CBF(133Xe 洗脱)的变化之间的关联。[66] 在 CBF 增加的情况下,包括 CO、动脉血压、中心静脉压和肺动脉舒张压在内的血液动力学变量仍然稳定。脑动静脉畸形具有独特的血流动力学生理特征,包括相对较低的跨壁压力梯度,可能通过病变部位分流一部分 CBF 。[67] 因此,据推测,手术切除后,原本通过动静脉畸形的那部分 CBF 重新通过正常大脑,在系统血流动力学不变的情况下,导致区域内 CBF 增加。
# 心脏手术
Cardiac Surgery
Cardiac surgery with cardiopulmonary bypass is a special situation in which organ perfusion is propelled by an extracorporeal centrifugal pump. How the pump flow affects the cerebral perfusion depends on the blood gas management.[68] With α-stat management, CBF (133Xe washout) is correlated with blood pressure, not pump flow.[69] With pH-stat management, CBF (argon saturation and desaturation method) is correlated with pump flow in the face of a stable blood pressure.[70] The precise mechanism(s) underlying this discrepancy is unclear. The cerebral vasodilation induced by hypercapnia may be responsible, because carbon dioxide is often added during pH-stat management but not during α-stat management.[71] Hypothermic cardiopulmonary bypass suppresses sympathetic nervous activity[72] and that may also alter the association between CO and CBF.
体外循环心脏手术是一种特殊情况,器官灌注是由体外离心泵推动的。泵流量如何影响脑灌注取决于血气管理。[68] 在 α- 状态管理下,CBF(133Xe 冲洗)与血压相关,而不是泵流量。[69] 在 pH - 状态管理下,CBF(氩饱和和去饱和法)在血压稳定的情况下与泵流量相关。[70] 这种差异的确切机制尚不清楚。高碳酸血症引起的脑血管扩张可能是原因,因为二氧化碳经常在 pH 值管理期间添加,但在 α 值管理期间不添加。[71] 低温体外循环抑制交感神经活动 [72],这也可能改变 CO 和 CBF 之间的联系。
# 肝衰竭
Hepatic Failure
An association between CO and CBF (133Xe washout) was not found in patients with fulminant hepatic failure.[73] However, this study was underpowered with only eight pairs of data, and the statistical insignificance likely reflects a single outlier. This study also found that the norepinephrine-induced changes in CO and TCD-estimated CBF did not correlate with each other. However, norepinephrine primarily increases blood pressure and has unpredictable effects on CO.[74] Therefore, the study was confounded by the simultaneous change in blood pressure.
在暴发性肝衰竭患者中没有发现 CO 和 CBF(133Xe 冲洗)之间的关联。[73] 然而,这项研究的效力不够,只有 8 对数据,统计学上的不显著性可能反映了一个单一的异常点。这项研究还发现,去甲肾上腺素引起的 CO 和 TCD 估计的 CBF 变化并不相互关联。然而,去甲肾上腺素主要是增加血压,对 CO 有不可预测的影响。[74] 因此,该研究被同时发生的血压变化所混淆。
# 心脏病
Cardiology
A study performed in patients with coronary heart disease or cardiomyopathy referred for echocardiography failed to show an association between CO and CBF.[75] However, the study was confounded by the use of common carotid artery blood flow measured using color M-mode duplex system as a surrogate for CBF as the external carotid artery blood flow is included.
冠心病或心肌病患者中进行的一项心脏超声研究未能显示 CO 和 CBF 之间的关联。[75] 然而,该研究被使用彩色 M 型双相系统测量的颈总动脉血流作为 CBF 的替代物所混淆,因为颈外动脉血流被包括在内。
# CBF 的综合调节
Integrated Regulation of CBF
CBF is rigorously regulated by multiple powerful mechanisms to safeguard the matching of cerebral metabolic demand and supply.[76] CO is one of the physiologic processes that contribute to CBF regulation. However, exactly how an alteration in CO, in the face of a stable blood pressure, leads to a change in CBF is not entirely clear. A proposal that integrates various CBF-regulating mechanisms, including the role of blood pressure and CO, in one concordant conceptualization seems necessary.
CBF 受到多种强大机制的严格调节,以保障脑代谢需求和供应的匹配。[76] CO 是有助于 CBF 调节的生理过程之一。然而,在血压稳定的情况下,CO 的改变究竟如何导致 CBF 的改变,目前还不完全清楚。似乎有必要提出一个建议,将各种 CBF 调节机制,包括血压和 CO 的作用,整合到一个统一的的概念中。
A conceptual framework of the integrated regulation of the brain perfusion is proposed ([fig. 3]). It needs to be appreciated that the various mechanisms, no matter how distinctive, all exert their regulatory effects on the same target, that is, the cerebral resistance vessels. Different mechanisms may affect different segments of the cerebral resistance vessels. For example, sympathetic stimulation constricts large cerebral arteries, whereas an increase in blood pressure constricts the arterioles.[77] The various CBF-regulating mechanisms integrate at the level of the cerebral resistance vessels and generate only one consequence that is the extent of the cerebrovascular resistance. Therefore, how CBF is changed after a change in any of the regulatory processes depends on how the different mechanisms are integrated. Different mechanisms likely have different degrees of regulatory power likely determined by the physiologic priority in the context of the clinical situation. The one with the major regulatory power plays a dominant role, whereas one with minor power plays a smaller role.
我们提出了一个脑灌注综合调节的概念框架([图 3])。需要理解的是,各种机制,无论多么独特,都对同一目标,即脑阻力血管产生调节作用。不同的机制可能影响大脑阻力血管的不同部分。例如,交感神经刺激使较大的脑动脉收缩,而血压升高则使小动脉血管收缩。[77] 各种 CBF 调节机制在脑阻力血管水平上整合,只产生一个后果,即脑血管阻力的程度。因此,在任何一个调节过程发生变化后,CBF 是如何变化的,取决于不同机制是如何整合的。不同的机制可能有不同程度的调节能力,可能由临床情况下的生理优先级决定。具有主要调节能力的机制起主导作用,而具有次要调节能力的机制则起较小的作用。
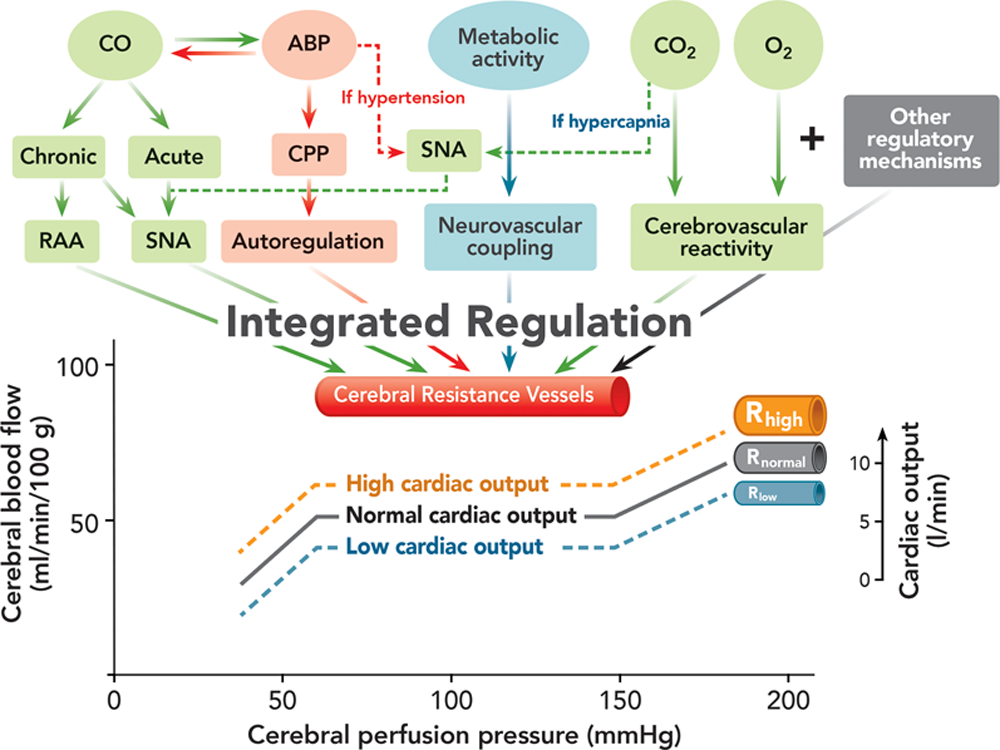
Fig. 3. The conceptual framework of the integrated regulation of brain perfusion. The cerebrovascular resistance determined by the caliber of the cerebral resistance vessels is regulated by various physiologic processes: (1) cardiac output (CO) likely via sympathetic nervous activity (SNA) and renin–angiotensin–aldosterone (RAA) system, depending on the chronicity of the change in CO,[5–19] (2) arterial blood pressure (ABP) and cerebral perfusion pressure (CPP) via cerebral autoregulation,[2],[71] (3) cerebral metabolic activity via neurovascular coupling,[3],[76] and (4) arterial blood carbon dioxide (CO2)[4],[25],[71] and oxygen (O2)[4] via cerebrovascular reactivity. The SNA regulates cerebral blood flow[26–29] and may play a prominent role during acute hypertension and hypercapnia[29] as a protective mechanism preventing cerebral overperfusion (dashed line). These various regulatory mechanisms, together with other CBF-regulatory mechanisms that are not specified here such as anesthetic effects, integrate at the level of the cerebral resistance vessels and generate only one consequence, which is the extent of the cerebrovascular resistance and, therefore, jointly regulate brain perfusion. The plateau of the autoregulation curve shifts downward when the CO is reduced and upward when augmented. The position of the plateau is determined by the caliber (R) of the cerebral resistance vessels at high (Rhigh), normal (Rnorm), and low (Rlow) CO. The scale of CO on the right side is smaller than that of CBF on the left side to reflect the lesser extent of change in CBF induced by an alteration of CO.
图 3. 脑灌注综合调节的概念框架。由脑阻力血管口径决定的脑血管阻力由各种生理过程调节:(1)心输出量(CO)可能通过交感神经活动(SNA)和肾素 - 血管紧张素 - 醛固酮(RAA)系统,取决于 CO 变化的慢性程度,[5-19](2)动脉血压(ABP)和脑灌注压(CPP)通过脑自主调节,[2],[71](3)脑代谢活动通过神经血管耦联,[3], [76] 和 (4) 动脉血二氧化碳(CO2)[4], [25], [71] 和氧(O2)[4] 通过 脑血管反应性。SNA 调节脑血流 [26-29],并可能在急性高血压和高碳酸血症期间发挥突出作用 [29],作为一种保护机制防止脑过度灌注(虚线)。这些不同的调节机制,连同其他 CBF 调节机制,如麻醉剂作用,在脑阻力血管水平上整合,只产生一个后果,即脑血管阻力的程度,因此共同调节脑灌注。当 CO 降低时,自动调节曲线的高点向下移动,当 CO 增加时,高点向上移动。平台的位置由高(Rhigh)、正常(Rnorm)和低(Rlow)CO 时的大脑阻力血管的口径(R)决定。右侧 CO 的比例小于左侧 CBF 的比例,以反映 CO 的改变所引起的 CBF 变化程度较小。
The effect of CO on CBF can be appreciated within the framework of cerebral autoregulation ([fig. 3]). When CO is decreased, the plateau descends slightly reflecting the smaller decrease in CBF, and vice versa; however, the overall autoregulatory mechanism is maintained. This proposition is corroborated by the finding that dynamic cerebral autoregulation is not affected by the acute change in CO.[78] Thus, this speculative proposal integrates the effects of blood pressure and CO on brain perfusion. However, how the lower and upper limits of the autoregulation curve are changed and whether the plateau tilts when the CO is altered are unknown.
在大脑自动调节的框架内,可以理解 CBF 的变化([图 3])。当 CO 降低时,平台略微下降,反映了 CBF 的较小下降,反之亦然;然而,整体的自动调节机制是保持的。[78] 因此,这一推测性建议整合了血压和 CO 对脑灌注的影响,证实了这一主张。然而,自动调节曲线的下限和上限是如何改变的,以及当 CO 改变时平台是否会倾斜,都是未知的。
The lesser extent to which CBF changes compared with that of CO or peripheral blood flow during acute or chronic CO alterations can be explained by the fact that the extent to which CBF is changed is determined by the integrated effect of all CBF-regulating mechanisms. Other powerful CBF-regulating mechanisms unrelated to CO may buffer the effect of CO on CBF, causing a lesser flow change in the brain compared with the organs that are not influenced by these mechanisms.
与 CO 或外周血流相比,在急性或慢性 CO 改变时,CBF 的变化程度较小,这可以解释为 CBF 的变化程度是由所有 CBF 调节机制的综合效应决定。其他与 CO 无关的强大的 CBF 调节机制可能会缓冲 CO 对 CBF 的影响,导致大脑中的流量变化比不受这些机制影响的器官要小。
# 临床意义
Clinical Implications
Acute changes in CO because of a variety of etiologies such as dehydration, blood loss, body tilt, mechanical ventilation, intraabdominal insufflation, pneumothorax, hemothorax, diuresis, vasodilation, sympatholysis, anesthetic agent, pulmonary embolization, myocardial infarction, and arrhythmia are frequently encountered in the operating room. CBF may decrease when the CO is reduced. Therefore, for the purpose of maintaining CBF, any adverse change in CO should be remedied. Goal-directed fluid therapy for the purpose of CO optimization has been shown to be associated with an improved overall outcome after intraabdominal surgeries.[79],[80] However, to what degree this favorable outcome is attributable to the optimization of CBF is unknown.
由于各种病因导致的 CO 急性变化,如脱水、失血、身体倾斜、机械通气、腹腔内充气、气胸、血胸、利尿、血管扩张、交感神经作用、麻醉剂、肺栓塞、心肌梗塞和心律失常等,在手术室经常遇到。当 CO 减少时,CBF 可能会减少。因此,为了维持 CBF,任何 CO 的不利变化都应该得到补救。为优化 CO 而进行的目标导向的液体治疗已被证明与腹腔内手术后的总体预后改善有关。[79], [80] 然而,这种较好的预后在多大程度上归因于 CBF 的优化是未知的。
It seems reasonable to advocate intraoperative monitoring of both CO and CBF in patients with reduced cardiac function or cerebrovascular obstructive diseases or during high-risk surgeries that have a greater chance of causing hemodynamic fluctuation. The currently unanswered question is how best to monitor both parameters continuously and noninvasively and which patient populations benefit the most from this strategy of care.
对于心脏功能减退或脑血管阻塞性疾病的患者,或在更有可能引起血流动力学波动的高风险手术中,提倡术中监测 CO 和 CBF 似乎是合理的。目前尚未解决的问题是如何最好地连续和无创地监测这两个参数,以及哪些患者群体从这种管理策略中受益最大。
In the perioperative setting, it needs to be emphasized that the cerebral circulation is affected by multiple processes, and CO is one of them. Anesthesia itself affects cerebral perfusion via a variety of pathways that include the suppression of cerebral metabolic activity,[81] intrinsic cerebral vasodilation by volatile agents,[82] impairment of cerebral autoregulation by volatile agents,[83] suppression of the sympathetic nervous activity,[84] and disturbance of the systemic hemodynamics.[85] Therefore, the association between CO and CBF learned from studies performed in unanesthetized healthy volunteers may not always apply in the anesthetized surgical patients.
在围手术期,需要强调的是,脑循环受到多种过程的影响,而 CO 是其中之一。麻醉本身通过各种途径影响脑灌注,包括抑制脑代谢活动,[81] 挥发性药剂的内在脑血管扩张,[82] 挥发性药剂对脑自动调节的损害,[83] 交感神经活动的抑制,[84] 和全身血流动力学的干扰。 [85] 因此,从未麻醉的健康志愿者的研究中了解到的 CO 和 CBF 之间的联系可能并不总是适用于麻醉的外科病人。
Chronic heart failure is prevalent affecting approximately 2% of the adult population and is associated with a high mortality.[86] Its prevalence increases sharply with age, affecting 10% of the population aged 65 yr or older.[87] An increasing number of patients diagnosed with chronic heart failure are expected to present to the operating room for surgery, and this poses a great challenge for perioperative care. It is judicious to avoid acute reductions of both CO and CBF on top of the chronic cardiac insufficiency and cerebral hypoperfusion. This mandates thoughtful preoperative preparation, adept appreciation of cardiovascular and cerebrovascular physiology and their interaction, and preemptively preventing circumstances that threaten cardiac performance and brain perfusion.
慢性心力衰竭是一种常见疾病,影响约 2% 的成年人群,与高死亡率相关。[86] 其发病率随着年龄的增长而急剧增加,影响到 65 岁或以上人口的 10%。[87] 越来越多的被诊断为慢性心力衰竭的病人预计会出现在手术室进行手术,这对围手术期的管理构成了巨大挑战。在慢性心功能不全和脑灌注不足的基础上,避免 CO 和 CBF 的急性降低是明智的。这就要求我们在术前进行周密的准备,熟练掌握心血管和脑血管生理学及其相互作用,并预先预防威胁心脏性能和脑灌注的情况。
Studies on the association between CO and CBF in patients with major neurologic, medical, or surgical conditions are confounded by methodologic limitations. However, it seems that interventions that enhance cardiac performance may improve perfusion of the ischemic brain, especially in patients with impaired cardiac function ([fig. 2]).[56–60] It is important although to remember that drugs that increase blood pressure such as phenylephrine and norepinephrine may actually decrease CO.[20],[74] In contrast, dobutamine and volume augmentation can increase the CO but not necessarily blood pressure. The effect of a vasopressor on CBF likely depends on the drug being used, the disease state, and the functional status of the regulatory mechanisms of brain perfusion.[88],[89] Currently, long-term outcome data relevant to the choice of vasopressor in various clinical situations is lacking.
对有重大神经系统、内科或外科疾病患者的 CO 和 CBF 之间关系的研究受到方法学限制的干扰。然而,提高心功能的干预措施似乎可以改善缺血脑的灌注,特别是在心脏功能受损的病人中([图 2])。[56-60] 但重要的是要记住,增加血压的药物,如苯肾上腺素和去甲肾上腺素,实际上可能减少 CO。血管抑制剂对 CBF 的影响可能取决于所使用的药物、疾病状态和脑灌注调节机制的功能状态。[88],[89] 目前,缺乏与在各种临床情况下选择血管抑制剂有关的长期结果数据。
The proposed conceptualization integrating various CBF-regulating mechanisms within the framework of cerebral autoregulation has important clinical implications. The habitual thinking that how the brain is perfused is merely dependent on the blood pressure should be abandoned. The autoregulatory curve should be regarded as a dynamic process, meaning that its shape, plateau, and the lower and upper limits may change depending on the integrated effect of nonpressure but CBF-regulating mechanisms including the CO.[71] For a given value of blood pressure, even though it is deemed clinically acceptable, the CBF may be either higher or lower than that estimated by the traditional autoregulatory curve. Therefore, the management of CBF should be guided by a multifactorial but integrated framework of CBF regulation, especially in patients who are at risk of cerebral ischemia.
提出的概念将各种 CBF 调节机制整合在大脑自动调节的框架内,具有重要的临床意义。应该摒弃大脑灌注方式仅仅取决于血压的习惯性思维。自动调节曲线应被视为一个动态过程,也就是说,其形状、平台以及下限和上限可能会根据包括 CO 在内的非压力但 CBF 调节机制的综合作用而改变。[71] 对于一个给定的血压值,即使它被认为是临床上可接受的,CBF 可能高于或低于传统自动调节曲线的估计值。因此,CBF 的管理应以多因素但综合的 CBF 调节框架为指导,尤其是对有脑缺血风险的患者。
Overall, these recommendations are largely based on physiologic studies in healthy volunteers and patients with chronic heart failure or other diagnoses. Meaningful outcome research pertinent to the management of CO and CBF is needed to better guide clinical practice. Moreover, noninvasive or minimally invasive, reliable, and continuous CO monitoring, as well as CBF monitoring or its surrogates, need to be considered for use in high-risk patients or during high-risk surgeries.
总的来说,这些建议主要是基于对健康志愿者和慢性心力衰竭或其他诊断患者的生理学研究。需要进行与 CO 和 CBF 管理相关的有意义的结果研究,以更好地指导临床实践。此外,无创或微创、可靠和连续的 CO 监测,以及 CBF 监测或其替代物,需要考虑用于高风险患者或高风险手术期间。
# 总结
Summary
As one of the most important systemic hemodynamic parameters, CO contributes to the regulation of CBF likely via the sympathetic nervous activity, with or without the renin–angiotensin system depending on the acuteness or chronicity of change. The various mechanisms that regulate the cerebral circulation integrate at the level of the cerebral resistance vessels and jointly determine the brain perfusion. The effect of CO on brain perfusion should be integrated into the framework of cerebral autoregulation. The clinical considerations are confounded by methodologic limitations. Interventions aimed at enhancing cardiac performance and improving brain perfusion need to be tested by relevant clinical outcomes research.
作为最重要的系统血流动力学参数之一,CO 有助于 CBF 的调节,可能是通过交感神经活动,伴或不伴肾素 - 血管紧张素系统,取决于急慢性改变。调节脑循环的各种机制在脑阻力血管水平上整合,共同决定脑灌注。 CO 对脑灌注的影响应被纳入脑自动调节的框架。临床上的考虑受到方法学有其局限性的影响。旨在提高心功能和改善脑灌注的干预措施,需要通过相关的临床结果研究来检验。
下体负压仓。图为为下半身压力室中身体卸载 [直立下半身正压(LBPP)] 和加载 [仰卧下半身负压(LBNP)] 原理的示意图。在垂直 LBPP 过程中,腰部密封的压差(P1 P2)产生浮力,并最终减少朝向水平跑步机的有效地面反作用力。仰卧 LBNP 以相反的方式工作,通过腰部密封的压差(P1 P2),产生吸力,产生朝向垂直跑步机的地面反作用力。(H.Ruckstuhl 提供的修改示意图。)
![Fig. 1. Pictured is a schematic view of the principles of body unloading [upright lower body positive pressure (LBPP)] and loading [supine lower body negative pressure (LBNP)] in a lower body pressure chamber. During upright LBPP, a pressure differential (P1 P2) across the waist seal creates a buoyancy force and ultimately reduces the effective ground reaction force toward the horizontal treadmill. Supine LBNP works the opposite way with a pressure differential (P1 P2) across the waist seal, creating a suction force that generates a ground reaction force toward the vertical treadmill. (Modified schematic drawing by courtesy of H. Ruckstuhl.)](/assets/img/2-Figure1-1.edf6de11.png) ↩︎
↩︎
